Toxicology
In case of accidental release of radionuclides into the environment, actinides represent a severe health risk to human beings after being incorporated by e.g. inhalation, ingestion or wounds. Contrary to other metal ions, actinides have no known essential function in biochemical reactions occurring in living organisms. The bioavailability and toxic effects depend strongly on the concentration and the speciation (chemical form, oxidation state) of the incorporated actinides. For a better understanding of the actinide behavior and their toxic effects in the human body (in term of metabolism, retention, and excretion), a fundamental knowledge on the mechanisms of relevant biochemical reactions in the body is needed which can be an important prerequisite for the design and success of potential decontamination therapies.
The interaction of actinides with blood serum proteins can play a major role in the biokinetic behavior of actinides after internal contamination. One representative of utmost importance is human serum albumin (HSA), the most abundant protein in the human blood plasma. Besides its contribution to the regulation of the colloid osmotic pressure, HSA binds and transports a range of predominantly water-insoluble molecules such as fatty acids, hormones, bilirubin, heme, drugs, as well as metal ions (such as Na+, Ca2+, Cu2+ and Zn2+). Although there is still a lack of detailed structural information, it is known that most mammalian albumins including HSA have at least four metal ion binding sites: the N-terminal Cu and Ni binding site (ATCUN site, also known as N-terminal site, NTS), the Multi-Metal Binding Site (MBS, also known as Site A), the site around Cys-34 and Site B, its location is still unknown. It is well-known that HSA forms complexes with a wide range of metal ions, especially transition metals. However, up to now only very few data regarding the interaction of lanthanides and actinides with albumin is reported.
Transferrin (fig. 1) is an iron carrier protein in blood. Transferrin can bind to two Fe(III) ions and transport them to the cells where the transferrin metal complex is recognized by the receptor and taken up via endocytosis. The ternary structure is characterized by folding into two similar lobes housing a metal binding site for Fe(III). In both lobes Fe(III) is coordinated by two tyrosines, one aspartate, one histidine and the synergistic anion carbonate in a distorted octahedral geometry. In blood, transferrin is about 30% saturated with iron. Consequently, non-saturated transferrin is available for the complexation of other metal ions. Besides iron, about 30 other tri- and tetravalent metal ions have been identified to bind to transferrin, e.g. Ga(III), Al(III), In(III), lanthanid(III) ions. Therefore, transferrin can significantly affect the biodistribution of metal ions in the living organism. Regarding the interaction of transferrin with actinides, a few studies have been performed with uranium, plutonium, and neptunium, whereas little information exists for the trivalent actinides, Am(III) and Cm(III).
Further proteins, which may affect the distribution of incorporated actinides and their internalization into cells, are hemoglobin and ferritin. Hemoglobin is the iron-containing oxygen-transport metalloprotein in the red blood cells (erythrocytes) of almost all vertebrates. Ferritin (fig. 2) is a globular protein complex consisting of 24 protein subunits forming a nanocage. Each ferritin complex can store up to 4500 Fe3+ ions as ferrihydrite.

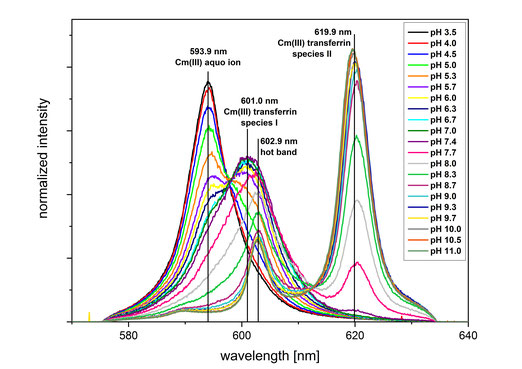
We study the interaction of proteins with trivalent actinides and lanthanides using advanced spectroscopic methods such as time-resolved fluorescence spectroscopy (TRLFS, fig. 3) and extended X-ray absorption fine-structure (EXAFS) spectroscopy. The complexation reactions are studied in dependence of pH, protein concentration, temperature and carbonate concentration to obtain detailed information on the speciation and coordination environment of the An(III)/Ln(III) protein complexes.